Antibodies against myositis associated antigens (IgG)
| ORDER NO. | ANTIBODIES AGAINST | IG CLASS | SUBSTRATE | FORMAT |
|---|---|---|---|---|
| DL 1530-1601-3 G | Mi-2, Ku, PM-Scl100, PM-Scl75, Jo-1, SRP, PL-7, PL-12, EJ, OJ und Ro-52 | IgG | Ag-coated immunoblot strips | 16 x 01 (16) |
Indications: Dermato- and polymyositis, idiopathic myositis, antisynthetase syndrome, overlapping sydrome.
Principles of the test: The EUROLINE test kit provides a qualitative in-vitro assay for human autoantibodies of the IgG class to 7 different antigens: Mi-2, Ku, PM-Scl100, PM-Scl75, Jo-1, SRP, PL-7, PL-12, EJ, OJ and Ro-52 in serum or plasma. The test kit contains test strips coated with parallel lines of highly purified antigens. In the first reaction step, diluted patient samples are incubated with the immunoblot strips. In the case of positive samples, the specific IgG antibodies (also IgA and IgM) will bind to the corresponding antigenic site. To detect the bound antibodies, a second incubation is carried out using an enzyme-labelled anti-human IgG (enzyme conjugate) which is capable of promoting a colour reaction.
Contents of the test kit:
| Component | Format | Symbol |
|---|---|---|
| 1. Test strips coated with the antigens: Mi-2, Ku, PM-Scl100, PM-Scl75, Jo-1, SRP, PL-7, PL-12, EJ, OJ and Ro-52 | 16 strips | STRIPS |
| 2. Positive control (IgG, human), 100x concentrate | 1 x 0.02 ml | POS CONTROL 100x |
| 3. Enzyme conjugate Alkaline phosphatase-labelled anti-human IgG (goat), 10x concentrate | 1 x 3 ml | .CONJUGATE 10x |
| 4. Sample buffer ready for use | 1 x 100 ml | .SAMPLE BUFFER |
| 5. Wash buffer 10x concentrate | 1 x 50 ml | WASH BUFFER 10x |
| 6. Substrate solution Nitrobluetetrazoliumchloride/5-Bromo-4-chloro-3-indolylphosphate (NBT/BCIP), ready for use | 1 x 30 ml | SUBSTRATE |
| 7. Evalution protocol | 1 sheet | |
| 8. Incubation tray | 2 x 8 channels | |
| 9. Plastic foil | 1 foil | |
| 10. Test instruction | 1 booklet |
Storage and stability: The test kit must be stored at a temperature between +2°C to +8°C. Do not freeze. Unopened, all test kit components are stable until the indicated expiry date.
Waste disposal: Patient samples, controls and incubated test strips should be handled as infectious waste. Other reagents do not need to be collected separately, unless stated otherwise in official regulations.
Preparation and stability of the reagents
Note: All reagents must be brought to room temperature (+18°C to +25°C) around 30 minutes before use. After first use, the reagents are stable until the indicated expiry date if stored at +2°C to +8°C and protected from contamination, unless stated otherwise below.
- Coated test strips: Ready for use. Open the package with the test strips only when the strips have reached room temperature to prevent condensation on the strips. After removal of the strips the package should be sealed tightly and stored at +2°C to +8°C.
- Positive control: The control is a 100x concentrate. For the preparation of the ready for use control the amount required should be removed from the bottle using a clean pipette and diluted 1:101 with sample buffer. Example: add 15 ?l of control to 1.5 ml of sample buffer and mix thoroughly. The ready for use diluted control should be used at the same working day.
- Enzyme conjugate: The enzyme conjugate is supplied as a 10x concentrate. For the preparation of the ready for use enzyme conjugate the amount required should be removed from the bottle using a clean pipette and diluted 1:10 with sample buffer. For one test strip, dilute 0.15 ml enzyme conjugate with 1.35 ml sample buffer. The diluted enzyme conjugate should be used at the same working day.
- Sample buffer: Ready for use.
- Wash buffer: The wash buffer is supplied as a 10x concentrate. For the preparation of the ready for use wash buffer the amount required should be removed from the bottle using a clean pipette and diluted 1:10 with distilled water. For one test strip, dilute 1 ml in 9 ml of distilled water. The ready-touse diluted wash buffer should be used at the same working day.
- Substrate solution: Ready for use. Close bottle immediately after use, as the contents are sensitive to light.
Warning: The controls used have tested negative for HBsAg, and antibodies against HCV, HIV-1 and HIV-2 using enzyme immunoassays or indirect immunofluorescence methods. Nonetheless all materials should be treated as being a potential infection hazard and should be handled with care. Some of the reagents are poisonous (buffer, substrate solution). Avoid contact with skin.
Preparation and stability of the patient samples
Sample material: Human serum or EDTA, heparin or citrate plasma.
Stability: Patient samples to be investigated can generally be stored at +2°C to +8°C for up to 14 days. Diluted samples should be incubated within one working day.
Sample dilution: The patient samples for analysis are diluted 1:101 with sample buffer. For example, add 15 ?l of serum to 1.5 ml sample buffer and mix well by vortexing. Sample pipettes are not suitable for mixing.
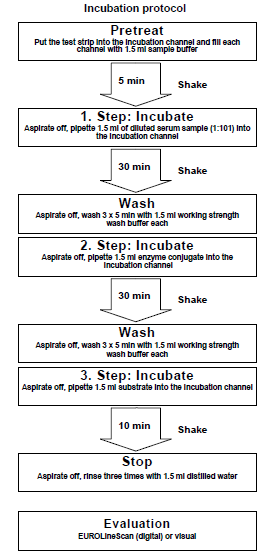
Incubation
| Pretreat: | Remove the required amount of test strips from the package and place them each in an empty channel. The number on the test strip should be visible. Fill the channels of the incubation tray according to the number of serum samples to be tested with 1.5 ml sample buffer each. Incubate for 5 minutes at room temperature on a rocking shaker. Afterwards aspirate off all the liquid. |
|---|---|
| Incubate: (1st step) | Fill each channel with 1.5 ml of the diluted serum samples and incubate at room temperature (+18°C to +25°C) for 30 minutes on a rocking shaker. |
| Wash: | Aspirate off the liquid from each channel and wash 3 x 5 minutes each with 1.5 ml working strength wash buffer on a rocking shaker. |
| Incubate: (2nd step) | Pipette 1.5 ml diluted enzyme conjugate (alkaline phosphatase-labelled antihuman IgG) into each channel and incubate for 30 minutes at room temperature (+18°C to +25°C) on a rocking shaker. |
| Wash: | Aspirate off the liquid from each channel. Wash as described above. |
| Incubate: (3rd step) | Pipette 1.5 ml substrate solution into the channels of the incubation tray. Incubate for 10 minutes at room temperature (+18°C to +25°C) on a rocking shaker. |
| Stop: | Aspirate off the liquid from each channel and wash each strip 3 x 1 minute with distilled water. |
| Evaluate: | Place test strip on the evaluation protocol, air dry and evaluate. |
For automated incubation with the EUROBlotMaster select the programme Euro01 AAK EL30.
EUROIMMUN Myositis Profile 3 EUROLINE (IgG)
Interpretation of Results
Handling: Place the wet strips after incubation onto the plastic foil on the evaluation protocol and align with the marks. Carefully dab the strips with absorbent paper (after complete drying the strips stick to the plastic foil). Distinctly recognizable bands on the dried blot strips which correspond with marks on the evaluation matrix are entered in the evaluation protocol. A white band at the position of an antigen has to be interpreted as negative. For long term stability the test strips should be coated with the included protective foil. If the evaluation is performed digitally using EUROLineScan, the blot strips should be placed onto the corresponding work sheet as described above. After scanning the dry blot strips can be coated with the adhesive plastic foil for longer stability. The code for entering the Test in the EUROLineScan is Myositis-EL 3.
There is a control band on the strips. The incubation was performed correctly if a strong colour reaction is visible on this control band.
Antigens and their arrangement on the strips: The EUROLINE test strips have been coated with the following antigens:
Mi-2: Recombinant Mi-2 protein. The corresponding human cDNA has been expressed with the baculovirus system in insect cells.
Ku: Recombinant Ku protein. The corresponding human cDNA has been expressed with the baculovirus system in insect cells.
PM-Scl100: Recombinant PM-Scl protein (100kDa). The corresponding human cDNA has been expressed with the baculovirus system in insect cells.
PM-Scl75: Recombinant PM-Scl protein (75kDa). The corresponding human cDNA has been expressed with the baculovirus system in insect cells.
Jo-1: Native Jo-1 protein (Histidyl-tRNA-synthetase), purified by affinity chromatography from calf and rabbit thymus.
SRP: Rekombinantes SRP Protein (54 kDa, signal recognition particle). The corresponding human cDNA has been expressed with the baculo-virus system in insect cells.
PL-7: Recombinant PL-7 protein (Threonyl-tRNA-synthetase). The corresponding human cDNA has been expressed with the baculovirus system in insect cells.
PL-12: Recombinant PL-12 protein (Alanyl-tRNA-synthetase). The corresponding human cDNA has been expressed with the baculovirus system in insect cells.
EJ: Recombinant EJ protein (Glycyl-tRNA-Synthetase). The corresponding human cDNA has been expressed in E. coli.
OJ: Recombinant OJ protein (Isoleucyl-tRNA-synthetase). The corresponding human cDNA has been expressed in E. coli.
Ro-52: Recombinant Ro-52 (52 kDa). The corresponding human cDNA has been expressed with the baculovirus system in insect cells.
Based on signal intensity, the results can be divided into negative, borderline and positive results.
| Signal Visual evaluation | Signal intensity EUROLineScan | Result |
|---|---|---|
| No signal | 0-5 | Negative |
| Very weak band | 6-10 | Borderline |
| Medium to strong band | 11-24 or 25-50 | Positive |
| Very strong band with an intensity comparable to the control band. | >50 | Strong positive |
An indirect immunofluorescence test should always be performed in parallel with the Myositis Profile EUROLINE. Autoantibodies detected using EUROLINE should be compared with the results of ANA screening tests (indirect immunofluorescence using the substrate combination HEp-2 cells and frozen sections of primate liver order no: FA 1510).
Only those results for which there is a plausible correspondence between the screening test (indirect immunofluorescence with the substrate combination HEp-2 cells, liver/monkey, order no.: FA 1510) and the confirmatory test should be evaluated. Antibodies against cytoplasmic antigens (Jo-1, SRP, PL-7, PL-12, EJ and OJ) are sometimes not clearly detectable with the IIFT screening. Parallel testing with screening and confirmatory test is recommended.
| Results of the screening test indirect immunofluorescence with the substrate combination HEp-2/liver | Characteristics Myositis Profile 3 EUROLINE | Result |
|---|---|---|
| ANA positive: Fine-granular fluorescence of cell nuclei, some nucleoli not visible. | Antigen band Mi-2 | Anti-Mi-2 positive |
| ANA positive ”Type Ku“: Fine granular fluorescence in the cell nuclei, nucleoli partly positive. Primate liver: Fine granular, partly reticular fluorescence in the cell nuclei. | Antigen band Ku | Anti-Ku positive |
| ANA positive nucleolar pattern: Homogeneous fluorescence of the nucleoli, at the same time a weaker, fine granular reaction in the nucleoplasm. Chromosomes of mitotic cells excluded, fine granular fluorescence outside of the chromosomes. Primate liver: Homogeneous fluorescence of the nucleoli. | Antigen band PM-Scl100 and/or PM-Scl75l | Anti-PM-Scl positive |
| Frequently granular to fine clumped fluorescence in the entire cytoplasm, cell nuclei sometimes with fine granula, more weakly expressed than in the cytoplasm. | Antigen band Jo-1 | Anti-Jo-1 positive |
| Frequently fine granular to homogeneous fluorescence in the cytoplasm. | Antigen band PL-7, Antigen band PL-12, Antigen band EJ, Antigen band OJ | Anti-PL-7 positive, Anti-PL-12 positive, Anti-EJ positive, Anti-OJ positive |
| ANA positive or negative | Antigen band Ro-52 | Anti-Ro-52 positive |
Isolated antibody reactions with Ro-52 should not be evaluated as anti-SS-A positive or specific for SLE or Sjögren’s syndrome, since they can occur in many different autoimmune diseases.
Test characteristics
Measurement range: The EUROLINE is a qualitative method. No measurement range is provided. The titre limit is given at a dilution of 1:101.
Cross reactions: The high analytical specificity of the test system is guaranteed by the quality of the antigen substrates used (antigens and antigen sources). This EUROLINE specifically detects IgG class antibodies to Mi-2, Ku, PM-Scl100, PM-Scl75, Jo-1, SRP, PL-7, PL-12, EJ, OJ and Ro-52. No cross reactions with other autoantibodies have been found.
Interference: Haemolytic, lipaemic and icteric sera up to a concentration of 5 mg/ml for haemoglobin, of 20 mg/ml for triglycerides and of 0.4 mg/ml bilirubin showed no effect on the analytical results of the present EUROLINE.
Inter- and intra-assay variation: The inter-assay variation was determined by multiple analyses of characterized samples over several days. The intra-assay variation was determined by multiple analyses of characterized samples on one day. In every case, the intensity of the bands was within the specified range. This EUROLINE displays excellent inter- and intra-assay reproducibility.
Clinical prevalences and specificity: In a study [23] performed at the University of Uppsala, Sweden, 153 sera from patients with clinically characterized myositis (50 patients with dermatomyositis, 89 patients with polymyositis, 4 patients with juvenile dermatomyositis and 10 patients with inclusion body myositis) as well as 77 sera from control patients (26 patients with Sjögren's syndrome, 26 patients with SLE and 25 patients with scleroderma) were tested for antibodies against Mi-2, Ku, PM-Scl100, Jo-1, PL-7 and PL-12. The prevalence values ranged between 3% and 12%, at a specificity for myositis of 97% to 100%. The total hit rate for antibodies against Mi-2, Ku, PM-Scl100, Jo-1, PL-7 and PL-12 was 26% in the myositis panel.
| Anti- | Prevalence | Specificity |
|---|---|---|
| Mi-2 | 3 % | 100 % |
| Ku | 3 % | 97 % |
| PM-Scl100 | 7 % | 100 % |
| Jo-1 | 12 % | 100 % |
| PL-7 | 2 % | 100 % |
| PL-12 | 0 % | 100 % |
A further study [24] carried out at the University of Padua, Italy showed similar results. In the investigation of 208 sera from patients with clinically characterized myositis and 214 sera from control patients (50 healthy persons, 13 patients with non-autoimmune myopathy, 23 sera from patients with CTD-associated myopathy, 65 patients with SLE, 34 patients with scleroderma, 21 patients with primary Sjögren’s syndrome, 8 patients with arthropathies) prevalence values of 4% to 21% were obtained, at a specificity for myositis of 95% to 100%. The total hit rate for antibodies against Mi-2, Ku, PM-Scl100, Jo-1, PL-7 and PL-12 was 37% in the myositis panel.
| Anti- | Prevalence | Specificity |
|---|---|---|
| Mi-2 | 4 % | 98 % |
| Ku | 5 % | 95 % |
| PM-Scl100 | 4 % | 100 % |
| Jo-1 | 21 % | 100 % |
| PL-7 or PL-12 | 4 % | 100 % |
In another study 194 patients with SLE, 131 patients with scleroderma, 179 patients with polymyositis/dermatomyositis (PM/DM) and 50 patients with rheumatoid arthritis (RA) were examined for antibodies against SRP, EJ, OJ and PM-Scl75. The prevalence of antibodies against SRP was 4%, at a specificity for myositis of 99%. The prevalence values for antibodies against EJ, OJ and PM-Scl75 ranged between 1% and 6% in the myositis and scleroderma panel, at a specificity for these diseases of 98% to 100%.
| Anti- | Prevalence | Specificity |
|---|---|---|
| SRP | 4 % | 99 % |
| EJ | 1 % | 100 % |
| OJ | 1 % | 100 % |
| PM-Scl75 | 6 % | 98 % |
Antibodies against Ro-52: Sera from 591 patients with rheumatic autoimmune diseases, from 260 patients with autoimmune and infectious liver diseases and from 50 healthy blood donors were tested for antibodies against Ro-52 using EUROLINE. Antibodies against Ro-52 are not associated with a specific disease, but they can be found in both autoimmune and infectious diseases with a prevalence of 5% to 81% [25].
| Disease | Prevalence of antibodies against Ro-52 | |
|---|---|---|
| Serum samples | Anti-Ro-52 positive (%) | |
| Sjögren´s syndrome | 88 | 81 |
| Scleroderma | 81 | 28 |
| Myositis | 26 | 31 |
| SLE | 210 | 38 |
| MCTD | 21 | 19 |
| Rheumatoid arthritis | 165 | 5 |
| Primary biliary liver cirrhosis | 100 | 27 |
| Autoimmune hepatitis | 60 | 35 |
| Hepatitis B | 50 | 10 |
| Hepatitis C | 50 | 22 |
| Healthy blood donors | 50 | 0 |
Clinical significance
Myositis is an inflammatory disease of the skeletal muscles. Myositides may be hereditary or caused by infections, malfunctions of the immune system or by toxins.
Polymyositis (PM) is an inflammatory disease of the skeletal muscles with perivascular lymphocytic infiltration. Its etiology is unknown. In case of skin involvement the disease is known as dermatomyositis (DM).
There are five different forms of polymyositis: primary idiopathic polymyositis (33% of cases), primary idiopathic dermatomyositis (33%), paraneoplastic dermatomyositis of the lungs, ovaries, mamma, gastrointestinal tract and in myeloproliferative diseases (8%), infantile dermatomyositis and accompanying vasculitis (5% to 10%) and myositis overlap syndrome in collagenoses (20%). Dermato-/polymyositis is often of paraneoplastic origin, particularly in elderly patients. Dermatomyositis symptoms can occur before the presence of the tumour is even diagnostically detectable.
Clinical symptoms of polymyositis are muscle weakness, unspecific inflammations, arthralgia, possibly Raynaud’s syndrome, trouble with swallowing and involvement of the inner organs. Dermatomyositis is characterized by the following skin manifestations: bluish-purple exanthema on the eyelids, nose bridge and cheeks, periorbital oedema, local erythema and scaling eczematous dermatitis.
Laboratory results show an increased level of muscle enzymes. The detection of myositis-associated autoantibodies with specific tests is essential in the diagnosis of dermato-/polymyositis, in controlling the course of the disease and in therapy management. Although the mortality rate is 4 times higher – with heart and lung diseases being the primary cause of death – half of the patients fully recover, however, a slight weakness of the muscles may remain. In 30% of cases, the disease can be stopped. 20% of patients experience deterioration despite therapeutic measures.
Antibodies against Mi-2 are highly specific for dermatomyositis. Antibodies against dermatomyositis can be found in 15% to 30% of patients. Antibodies against Mi-2 can also be detected in 8% to 12 % of patients with idiopathic myositis.
Antibodies against Ku have a prevalence of up to 10% in systemic lupus erythematosus (SLE), a systemic autoimmune disease belonging to the group of collagenoses which predominantly manifests itself by the so-called malar or butterfly rash. 40% of symptoms in patients with antibodies against Ku are myositis-related or characteristic of systemic sclerosis (SSc), a chronic autoimmune disease with fibrosis of the skin (sclerodermia), the joints and inner organs such as oesophagus, lungs, heart and kidneys.
Antibodies against PM-Scl (PM-1) are found in 50% to 70% of patients diagnosed with overlap syndrome: The disease manifests itself by a combination of polymyositis, dermatomyosits and systemic sclerosis (SSc) symptoms.
Antibodies against Jo-1 are found in polymyositis with a prevalence of 25% to 55%. They are often associated with simultaneously occurring autoimmune diseases such as SLE, SSc or interstitial lung fibrosis, an inflammatory response of the lung tissue with accompanying formation of connective tissue between the alveoli and the surrounding blood vessels.
Antibodies against PL-7 have a prevalence of approx. 3% to up to 6% in myositis patients, partly overlapping with SLE, SSc or interstitial lung fibrosis.
The prevalence of antibodies against PL-12 in myositis patients is up to 3%.

