Stomach (Rat) Instructions for the indirect immunofluorescence test
| ORDER NO. | ANTIBODIES AGAINST | SUBSTRATE | SPECIES | FORMAT SLIDES x FIELDS |
|---|---|---|---|---|
| FA 1710-0405 | 04 x 05 (020) | |||
| FA 1710-1003 | 10 x 03 (030) | |||
| FA 1710-1005 | 10 x 05 (050) | |||
| FA 1710-1010 | smooth muscles (ASMA) | stomach | rat | 10x 10 (100) |
| FA 1710-2005 | 20 x 05 (100) | |||
| FA 1710-2010 | 20 x 10 (200) | |||
| FA 1710-5010 | 50 x 10 (500) | |||
| FA 1710-0010 | 100 x 10 (1000) |
Test principle: This test kit is designed exclusively for the in vitro determination of human antibodies in serum or plasma. The determination can be performed qualitatively or quantitatively.
Frozen sections of rat stomach covering the reaction areas of a BIOCHIP Slide are incubated with diluted patient samples. If the reaction is positive, specific antibodies of classes IgA, IgG and IgM attach to the smooth muscle antigens. In a second step, the attached antibodies are stained with fluorescein-labelled anti-human antibodies and made visible with the fluorescence microscope.
Contents of a test system for 50 determinations:
| Description | Format | Symbol |
|---|---|---|
| 1. Slides, each containing 5 x 2 BIOCHIPs coated with frozen sections of rat stomach | 10 slides | SLIDE |
| 2. Fluorescein-labelled anti-human IgAGM (goat), ready for use | 1 x 1.5 ml | CONJUGATE |
| 3. Positive control: autoantibodies against smooth muscles (ASMA), human, ready for use | 1 x 0.1 ml | POS CONTROL |
| 4. Negative control: autoantibody negative, human, ready for us | 1 x 0.1 ml | NEG CONTROL |
| 5. Salt for PBS pH 7.2 | 2 packs | PBS |
| Tween 20 | 2 x 2.0 ml | TWEEN 20 |
| 7. Embedding medium, ready for use | 1 x 3.0 ml | GLYCEROL |
| 8. Cover glasses (62 mm x 23 mm) | 12 piece | COVERGLASS |
| 9. Instruction booklet | 1 booklet | --- |
Single slides (e.g., EUROIMMUN Order No. FB 1710-1005) are provided together with cover glasses. Additional positive control (e.g., Order No. CA 1710-0101) and negative control (e.g., Order No. CA 1000-0101 Z) can be ordered. Performance of the test requires reagent trays TRAY, which are not provided in the test kits. They are available from EUROIMMUN under the following order no.: - ZZ 9999-0110 for slides containing up to 10 fields
Storage and stability: The slides should be stored at -40°C (not below) to +8°C and the reagents at a temperature between +2°C and +8°C. Stability is guaranteed for 18 months after the date of manufacture if stored properly.
Waste disposal: Patient samples, controls and slides are to be handled as potentially infectious materials. All reagents are to be disposed in accordance with official disposal regulations.
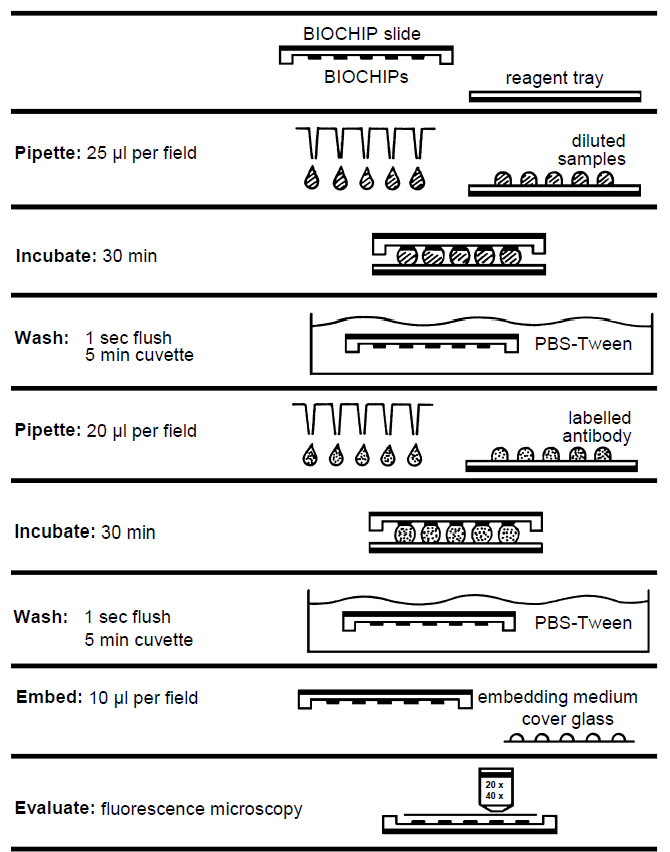
Performing the test (reaction fields 5 x 5 mm)
The TITERPLANE Technique was developed by EUROIMMUN in order to standardize immunological analyses: Samples or labelled antibodies are applied to the reaction fields of a reagent tray. The BIOCHIP Slides are then placed into the recesses of the reagent tray, where all BIOCHIPs of the slide come into contact with the fluids, and the individual reactions commence simultaneously. Position and height of the droplets are exactly defined by the geometry of the system. As the fluids are confined to a closed space, there is no need to use a conventional “humidity chamber”. It is possible to incubate any number of samples next to each other and simultaneously under identical conditions.
| Prepare: | The preparation of the reagents and of the serum and plasma samples is described on page 4 of this test instruction. |
|---|---|
| Pipette: | Apply 25 µl of diluted sample to each reaction field of the reagent tray, avoiding air bubbles. Transfer all samples to be tested before starting the incubation (up to 200 droplets). Use a polystyrene pipetting template. |
| Incubate: | Start reactions by fitting the BIOCHIP Slides into the corresponding recesses of the reagent tray. Ensure that each sample makes contact with its BIOCHIP and that the individual samples do not come into contact with each other. Incubate for 30 min at room temperature (+18°C to +25°C). |
| Wash: | Rinse the BIOCHIP Slides with a flush of PBS-Tween using a beaker and immerse them immediately afterwards in a cuvette containing PBS-Tween for at least 5 min . Shake with a rotary shaker if available. |
| Pipette: | Apply 20 µl of fluorescein-labelled anti-human globulin to each reaction field of a clean reagent tray. Add all droplets before continuing incubation. Use a stepper pipette. The labelled anti-human serum should be mixed with a pipette before use. To save time, conjugate can be pipetted onto separate reagent trays during the incubation with the diluted sample. |
| Incubate: | Remove one BIOCHIP Slide from cuvette. Within five seconds blot only the back and the long sides with a paper towel and immediately put the BIOCHIP Slide into the recesses of the reagent tray. Do not dry the areas between the reaction fields. Check for correct contact between the BIOCHIPs and liquids. Then continue with the next BIOCHIP Slide. From now on, protect the slides from direct sunlight. Incubate for 30 min at room temperature (+18°C to +25°C). |
| Wash: | Rinse the BIOCHIP Slides with a flush of PBS-Tween using a beaker and put them into a cuvette with PBS-Tween for at least 5 min . Shake with a rotary shaker if available. 10 drops of Evans Blue for each 150 ml phosphate buffer can be added for counterstaining. |
| Embed: | Place embedding medium onto a cover glass - drops of 10 µl per reaction field Use a polystyrene embedding template. Remove one BIOCHIP Slide from PBS- Tween and dry the back, all four sides, as well as the surface around, but not between the reaction fields with a paper towel. Put the BIOCHIP Slide, with the BIOCHIPs facing downwards, onto the prepared cover glass. Check immediately that the cover glass is properly fitted into the recesses of the slide. Correct the position if necessary. |
| Evaluate: | Read the fluorescence with the microscope. Objective for organ sections 20x, for infected cells 20x, for cell substrates 40x. Excitation filter: 488 nm, color separator: 510 nm, blocking filter: 520 nm Light source: mercury vapor lamp, 100 W, EUROIMMUN LED, EUROStar Bluelight |
TITERPLANE Technique
Preparation and stability of reagents
Note: The individual reagents of one lot are matched with one another and should not generally be swapped with reagents of another lot. After initial opening, the reagents are stable until the expiry date when stored between +2°C and +8°C and protected from contamination, unless stated otherwise below.
- Slides: Ready for use. Remove the protective cover only when the slides have reached room temperature (condensed water can damage the substrate). Mark with a felt-tip pen. Do Note touch the BIOCHIPs. After the protective cover has been opened, the slide should be incubated within 15 minutes. If the protective cover is damaged, the slide must not be used for diagnostics.
- Fluorescein-labelled secondary antibody (FITC): Ready for use. Before using FITC for the first time, mix thoroughly with a pipette. The conjugate is sensitive to light. Protect from sunlight.
- Positive and negative controls: Ready for use. Before using control sera for the first time, mix them thoroughly with a pipette.
- PBS-Tween: 1 pack of “Salt for PBS” should be dissolved in 1 liter of distilled water (optimal: aqua pro infusione, aqua ad injectabilia) and mixed with 2 ml of Tween 20. The prepared PBS-Tween can be stored at +2°C to +8°C, generally for 1 week. PBS-Tween should not be used if the solution becomes cloudy or contamination appears.
- Embedding medium: Ready for use.
- Reagent trays: Reaction fields of the reagent tray must be hydrophilic and surrounding area hydrophobic. If necessary, wipe with Extran MA 01 (Merck) and rinse generously with water. To disinfect: Immerse in Sekusept Extra (Henkel) (3% in water) for 1 hour. After disinfection rinse generously with water and dry with absorbent paper.
Warning: The BIOCHIPs coated with antigen substrates have been treated with a disinfecting fixing agent. Neither HBsAg nor antibodies against HIV-1, HIV-2, and HCV could be detected in the control sera using appropriate ELISA or indirect immunofluorescence tests. Nevertheless, all test system components should be handled as potentially infectious materials. Some of the reagents also contain the toxic agent sodium azide. Avoid contact with the skin.
Preparation and stability of serum and plasma samples
Samples: Human sera or EDTA, heparin or citrate plasma.
Stability: The patient samples to be investigated can generally be stored up to 14 days at a temperature between +2°C and +8°C. Diluted samples must be incubated within one working day.
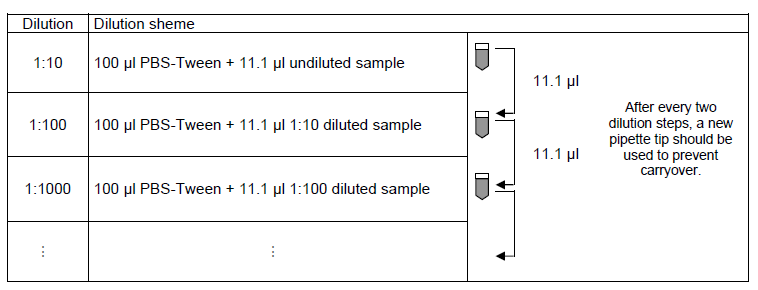
Recommended sample dilution for qualitative evaluation: The sample to be investigated is diluted 1:100 in PBS-Tween. For example, dilute 10.1 µl sample in 1000 µl PBS-Tween and mix thoroughly, e.g., vortex for 4 seconds.
Recommended Serum dilution for quantitative evaluation: The dilution of samples to be investigated is performed using PBS-Tween. For each add 100 µl of PBS-Tween to the tube and mix with 11.1 µl of the next highest concentration, e.g., vortex for 2 seconds. EUROIMMUN recommends incubating samples from a dilution of 1:100.
Test evaluation
Fluorescence pattern (positive reaction): With frozen sections of rat stomach antibodies against smooth muscles show a distinct cytoplasmic fluorescence of the tunica muscularis as well as the lamina muscularis mucosa and the interglandular contractile fibrils of the tunica mucosa.
If the positive control shows no specific fluorescence pattern or the negative control shows a clear specific fluorescence the results are not to be used and the test is to be repeated.
A large range of fluorescence images can be found on the EUROIMMUN website (www.euroimmun.com)
Qualitative evaluation:
| ASMA reactivity | Evaluation |
|---|---|
| No reaction at 1: 100 | Negative. No antibodies against smooth muscles detected in the patient samples. |
| Positive reaction at 1:100 | Positive. Indication of hronic-active hepatitis, viral hepatitis, infectious mononucleosis and others. |
Quantitative evaluation: The titer is defined as the sample dilution factor for which specific fluorescence is just identifiable. This should be compared with the reaction obtained using an equivalently diluted negative serum.
Antibody titers can be determined according to the following table from the fluorescence of the different sample dilutions.
| Fluorescence at | antibody titer | |||
| 1:10 | 1:100 | 1:1000 | 1:10.000 | |
| weak | negative | negative | negative | 1:10 |
| moderate | negative | negative | negative | 1:32 |
| strong | weak | negative | negative | 1:100 |
| strong | moderate | negative | negative | 1:320 |
| strong | strong | weak | negative | 1:1000 |
| strong | strong | moderate | negative | 1:3200 |
| strong | strong | strong | weak | 1:10,000 |
| : | : | : | : | : |
For diagnosis the clinical symptoms of the patient should always be taken into account alongside the serological results.
Test characteristics
Antigen: For the determination of autoantibodies against smooth muscles (ASMA) by means of indirect immunofluorescence, frozen tissue sections from various organs can be used, rat stomach being the standard substrate. Among others, oesophagus and intestinal tissue or the kidney of various species with a muscle layer or an adequate number of arteries are suitable. Some of the antibodies against smooth muscles are targeted towards actin and are tested using the substrate combination with HEp-2 cells and primate liver (EUROIMMUN Order No. FA 1510-1010-1).
Stability: Stability is guaranteed for 18 months after the date of manufacture if stored properly.
Measurement range: The dilution starting point for this measurement system is 1:100. Samples can be further diluted by a factor of 10 so that the dilution series is 1:1000, 1:10000 etc. There is no upper limit to the measurement range.
Intra-assay reproducibility: Inter-lot reproducibility was tested with more than 10 different lots. In quantitative evaluation of results, the deviation amounted to no more than ± 1 fluorescence intensity level. Intra-assay reproducibility can therefore also be guaranteed. The intensity of the specific fluorescence as a numeric value is called fluorescence intensity level by EUROIMMUN. These values can reach from „0“ (no specific fluoescence) to „5“ (extreme strong specific fluorescence).
Inter-assay reproducibility: Inter-lot reproducibility was tested with more than 10 different lots. In quantitative evaluation of results, the deviation amounted to no more than ± 1 fluorescence intensity level. Inter-assay reproducibility can therefore also be guaranteed.
Inter-lot reproducibility: Inter-lot reproducibility was tested with more than 10 different lots. In quantitative evaluation of results, the deviation amounted to no more than ± 1 fluorescence intensity level.
Cross reactivity: There is no data known to EUROIMMUN in which cross reactivities are described.
Interference: Hemolytic, lipemic and icteric samples showed no interferences.
Reference range: Titer 1: < 100>
Specificity: The specificity of the testsystem is 100%. A collective of n=36 characterized sera was investigated. The sera (Origin: Germany) were made available by the Institute for standardisation and documentation in the medical laboratory (INSTAND).
Sensitivity: The sensitivity of the testsystem is 100%. A collective of n=36 characterized sera in context of INSTAND*-Interlaboratory was investigated. The sera (Origin: Germany) were made available by the Institute for standardisation and documentation in the medical laboratory (INSTAND).
Clinical significance
Autoantibodies against smooth muscles (ASMA) occur in various liver diseases (autoimmune hepatitis, liver cirrhosis). Their determination is of particular significance for the diagnosis of autoimmune (lupoid) chronic active hepatitis. ASMA can also be found in infectious mononucleosis and other virus infections, in systemic lupus erythematosus, in breast and ovarian carcinomas as well as in malignant melanomas, but play no diagnostic role in these diseases. In the case of viral hepatitis the titer generally falls off again rapidly.
High concentrations of antibodies against smooth muscles are an indication of autoimmune hepatitis (AIH), the prevalence being 70%. The IgG and IgM titers can correlate with the disease activity. AIH occurs mostly in women, and half of the cases develop before the age of 30. In 40% of patients, the disease commences with acute hepatitis. Liver biopsies show a necrosis of the parenchymal cells with lymphocyte and plasma cell infiltration.
With the help of autoantibodies and various virus parameters, autoimmune hepatitis can be divided into various etiologic subgroups. In the case of AIH, autoantibodies against cell nuclei, double-stranded DNA and granulocyte cytoplasm (pANCA) can frequently also be found along with antibodies against smooth muscles.
Low ASMA titers are also detected in patients with primary biliary liver cirrhosis (50%), alcohol-related liver cirrhosis, obstruction of the biliary ducts and in about 2% of apparently healthy persons.