Antibodies against nuclear antigens (IgG)
Test instructions for the ANA Profile et Mi-2 et Ku EUROLINE
| ORDER NO. | ANTIBODIES AGAINST | IG-CLASS | SUBSTRATE | FORMAT |
|---|---|---|---|---|
| DL 1590-1601-31 GDL 1590-6401-31 G | Mi-2, Ku, nRNP/Sm, Sm, SS-A, Ro-52, SS-B, Scl-70, PM-Scl100, Jo-1, CENP B, PCNA, dsDNA, nucleosomes, histones, rib. P-prot., AMA M2 | IgG | Ag-coated immunoblot strips | 16 x 01 (16)64 x 01 (64) |
Indications: Sharp syndrome (MCTD), lupus erythematosus disseminatus (SLE), Sjögren's syndrome, progressive systemic sclerosis, poly-/dermatomyositis, overlap syndrome, limited form of progressive systemic sclerosis (CREST syndrome), primary biliary liver cirrhosis.
Principles of the test: The EUROLINE test kit provides a qualitative in vitro assay for human autoantibodies of the IgG class to 17 different antigens: Mi-2, Ku, nRNPSm, Sm, SS-A (native), Ro-52, SS-B, Scl-70, PM-Scl100, Jo-1, CENP B, PCNA, dsDNA, nucleosomes, histones, ribosomal P-protein and AMA M2 in serum or plasma. The test kit contains test strips coated with parallel lines of highly purified antigens. In the first reaction step, diluted patient samples are incubated with the immunoblot strips. In the case of positive samples, the specific IgG antibodies (also IgA and IgM) will bind to the corresponding antigenic site. To detect the bound antibodies, a second incubation is carried out using an enzyme-labelled anti-human IgG (enzyme conjugate) catalysing a colour reaction.
Contents of the test kit:
| Component | Format | Format | Symbol |
|---|---|---|---|
| 1. Test strips coated with the antigens: Mi-2, Ku, nRNP/Sm, Sm, SS-A (native), Ro-52, SS-B, Scl-70, PM-Scl100, Jo-1, CENP B, PCNA, dsDNA, Nucleosomes, Histones, rib. P-protein, AMA M2 | 16 strips | 4 x 16 strips | STRIPS |
| 2. Positive control (IgG, human), 100x concentrate | 1 x 0.02 ml | 4 x 0.02 ml | POS CONTROL 100x |
| 3. Enzyme conjugate Alkaline phosphatase-labelled anti-human IgG (goat), 10x concentrate | 1 x 3 ml | 4 x 3 ml | CONJUGATE 10x |
| 4. Sample buffer ready for use | 1 x 100 ml | 3 x 100 ml | SAMPLE BUFFER |
| 5. Wash buffer 10x concentrate | 1 x 50 ml | 1 x 100 ml | WASH BUFFER 10x |
| 6. Substrate solution Nitro blue tetrazolium chloride/5-Bromo-4-chloro-3-indolyl phosphate (NBT/BCIP), ready for use | 1 x 30 ml | 4 x 30 ml | SUBSTRATE |
| 7. Incubation tray | 2 x 8 channels | --- | |
| 8. Test instruction | 1 booklet | 1 booklet |
The following components are not provided in the test kits but can be ordered at EUROIMMUN under the respective order numbers.
Performance of the test requires an incubation tray:
- ZD 9899-0130 Incubation tray with 30 channels
- ZD 9898-0130 Incubation tray with 30 channels (black, for EUROBlotCamera system)
- ZD 9898-0148 Incubation tray with 48 channels (black, for EUROBlotCamera system)
For the creation of work protocols and the evaluation of incubated test strips using EUROLineScan green paper and adhesive plastic foil are required:
- ZD 9880-0101 Green paper (1 sheet)
- ZD 9885-0116 Adhesive foil for approx. 16 test strips
- ZD 9885-0130 Adhesive foil for approx. 30 test strips
If you wish to perform a visual evaluation, you may order the required evaluation protocol under:
- ZD 1590-0101-31 G Evaluation protocol visual ANA Profile et Mi-2 et Ku EUROLINE.
Storage and stability: The test kit must be stored at a temperature between +2°C to +8°C. Do not freeze. Unopened, all test kit components are stable until the indicated expiry date.
Waste disposal: Patient samples, controls and incubated test strips should be handled as infectious waste. Other reagents do not need to be collected separately, unless stated otherwise in official regulations.
Preparation and stability of the reagents
Note: All reagents must be brought to room temperature (+18°C to +25°C) approx. 30 minutes before use. Unopened, reagents are stable until the indicated expiry date when stored at +2°C to +8°C. After initial opening, reagents are stable for 12 months or until the expiry date, if earlier, unless stated otherwise in the instructions. Opened reagents must also be stored at +2°C to +8°C and protected from contamination.
- Coated test strips: Ready for use. Open the package with the test strips only when the strips have reached room temperature to prevent condensation on the strips. After removal of the strips the package should be sealed tightly and stored at +2°C to +8°C.
- Positive control: The control is a 100x concentrate. For the preparation of the ready for use control the amount required should be removed from the bottle using a clean pipette and diluted 1:101 with sample buffer. Example: add 15 µl of control to 1.5 ml of sample buffer and mix thoroughly. The ready for use diluted control should be used at the same working day.
- Enzyme conjugate: The enzyme conjugate is supplied as a 10x concentrate. For the preparation of the ready for use enzyme conjugate the amount required should be removed from the bottle using a clean pipette and diluted 1:10 with sample buffer. For one test strip, dilute 0.15 ml enzyme conjugate with 1.35 ml sample buffer. The diluted enzyme conjugate should be used at the same working day.
- Sample buffer: Ready for use.
- Wash buffer: The wash buffer is supplied as a 10x concentrate. For the preparation of the ready for use wash buffer the amount required should be removed from the bottle using a clean pipette and diluted 1:10 with distilled water. For one test strip, dilute 1 ml in 9 ml of distilled water. The ready for use diluted wash buffer should be used at the same working day.
- Substrate solution: Ready for use. Close bottle immediately after use, as the contents are sensitive to light.
Warning: The controls used have tested negative for HBsAg, and antibodies against HCV, HIV-1 and HIV-2 using enzyme immunoassays or indirect immunofluorescence methods. Nonetheless all materials should be treated as being a potential infection hazard and should be handled with care. Some of the reagents are poisonous (buffer, substrate solution). Avoid contact with skin.
Preparation and stability of the patient samples
Sample material: Human serum or EDTA, heparin or citrate plasma.
Stability: Patient samples to be investigated can generally be stored at +2°C to +8°C for up to 14 days. Diluted samples should be incubated within one working day.
Sample dilution: The patient samples for analysis are diluted 1:101 with sample buffer. For example, add 15 µl of serum to 1.5 ml sample buffer and mix well by vortexing. Sample pipettes are not suitable for mixing.
Incubation
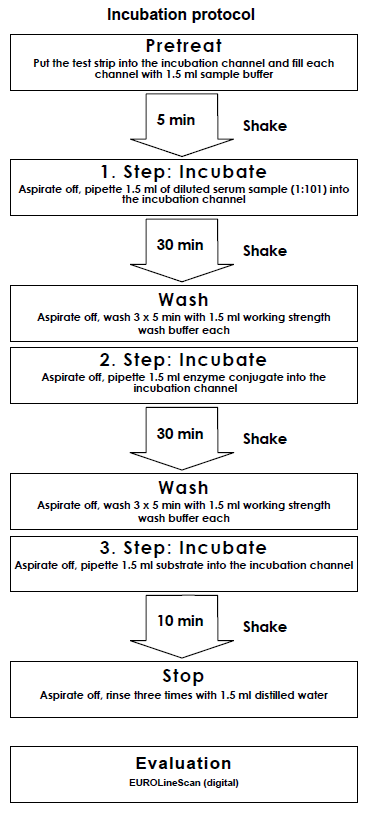
| Pretreat: | Remove the required amount of test strips from the package and place them each in an empty channel. The number on the test strip should be visible. Fill the channels of the incubation tray according to the number of serum samples that should be tested with 1.5 ml sample buffer each. Incubate for 5 minutes at room temperature on a rocking shaker. Afterwards aspirate off all the liquid. |
| Incubate: (1st step) | Fill each channel with 1.5 ml of the diluted serum samples and incubate at room temperature (+18°C to +25°C) for strong>30 minutes on a rocking shaker. |
| Wash: | Aspirate off the liquid from each channel and wash 3 x 5 minutes each with 1.5 ml working strength wash buffer on a rocking shaker. |
| Incubate: (2nd step) | Pipette 1.5 ml diluted enzyme conjugate (alkaline phosphatase-labelled anti-Human IgG) into each channel and incubate for 30 minutes at room temperature (+18°C to +25°C) on a rocking shaker. |
| Wash: | Aspirate off the liquid from each channel. Wash as described above. |
| Incubate: (3rd step) | Pipette 1.5 ml substrate solution into the channels of the incubation tray. Incubate for 10 minutes at room temperature (+18°C to +25°C) on a rocking shaker. |
| Stop: | Aspirate off the liquid from each channel and wash each strip 3 x 1 minute with distilled water. |
| Evaluate: | Place test strip on the evaluation protocol, air dry and evaluate. |
For automated incubation with the EUROBlotMaster select the programme Euro01 AAb EL30.
EUROIMMUN ANA Profile et Mi-2 et Ku EUROLINE (IgG)
Interpretation of Results
Handling: For the evaluation of incubated test strips we generally recommend using the EURO-LineScan software. After stopping the reaction using deionised or distilled water, place the incubated test strips onto the adhesive foil of the green work protocol using a pair of tweezers. The position of the test strips can be corrected while they are wet. As soon as all test strips have been placed onto the protocol, they should be pressed hard using filter paper and left to air-dry. After they have dried, the test strips will be stuck to the adhesive foil. The dry test strips are then scanned using a flatbed scanner (EUROIMMUN AG) and evaluated with EUROLineScan. For general information about the EUROLineScan programme please refer to the EUROLineScan user manual (EUROIMMUN AG). The code for entering the test into EUROLineScan is AnaMK.
If a visual evaluation must be performed, place the incubated test strips onto the respective work protocol for visual evaluation. This protocol is available at EUROIMMUN under the order no. ZD 1590-0101-31 G.
There is a control band on the strips. The incubation was performed correctly if a strong colour reaction is visible on this control band. A white band at the position of an antigen has to be interpreted as negative.
Antigens and their arrangement on the strips: The EUROLINE test strips have been coated with the following antigens:
Mi-2: Recombinant Mi-2 protein. The corresponding human cDNA has been expressed with the baculovirus system in insect cells.
Ku: Recombinant Ku protein. The corresponding human cDNA has been expressed with the baculovirus system in insect cells.
nRNP/Sm: Native U1-nRNP purified by affinity chromatography from calf and rabbit thymus.
Sm: Native Sm antigen purified by affinity chromatography from The Sm antigen contains the core proteins of snRNP particles. D protein is the main component of the Sm preparation.
SS-A: Native SS-A antigen purified by affinity chromatography from bovine spleen and thymus.
Ro-52: Recombinant Ro-52 (52 kDa). The corresponding human cDNA has been expressed with the baculovirus system in insect cells.
SS-B: Native SS-B antigen purified by affinity chromatography from calf and rabbit thymus.
Scl-70: Native Scl-70 (DNA-Topoisomerase I) antigen purified by affinity chromatography from bovine and rabbit thymus.
PM-Scl: Recombinant PM-Scl100. The corresponding human cDNA has been expressed with the baculovirus system in insect cells.
Jo-1: Native Jo-1 (Histidyl-tRNA Synthetase) antigen purified by affinity chromatography from calf and rabbit thymus.
CENP B: Recombinant centromere protein B. The corresponding human cDNA has been expressed with the baculovirus system in insect cells.
PCNA: Recombinant PCNA (36kDa). The corresponding human cDNA has been expressed with the baculovirus system in insect cells.
dsDNA: Highly purified native, double-stranded DNA isolated from salmon testes.
Nucleosomes: Native nucleosomes purified from calf thymus.
Histones: A mixture of individually purified histone types isolated from calf thymus.
Rib. P-protein: Native ribosomal P-proteins purified by affinity chromatography from calf and rabbit thymus.
AMA-M2: Native M2 antigen (pyrutate-dehydrogenase complex) purified from pork heart.
EUROIMMUN recommends interpreting results based on the signal intensity:
| Signal Visual evaluation | Signal intensity EUROLineScan | Result | |
|---|---|---|---|
| No signal | 0-5 | 0 | Negative |
| Very weak band | 6-10 | (+) | Borderline |
| Medium to strong band | 11-25 or 26-50 | +, ++ | Positive |
| Very strong band with an intensity comparable to the control band | >50 | +++ | Strong positive |
Results in the borderline range from 6 to 10 should be evaluated as increased but negative.
An indirect immunofluorescence test should always be performed in parallel with the determination of cell nucleus antibodies by EUROLINE. On the one hand, this provides a check on plausibility as a safeguard against false-positive results, on the other hand, by using EUROIMMUN HEp-2 cells, and in particular in combination with frozen sections of primate liver, immunofluorescence permits the detection of a wider range of cell nucleus antibodies, as not all cell nucleus antigens are presently available in the EUROLINE.
Test characteristics
Calibration: The reactivity of each antigen is standardized by the human reference sera CDC-ANA #1 to #11 of the "Center for Disease Control" (Atlanta, USA). The reactivity of the CDC sera in the EUROIMMUN ANA Profile EUROLINE is summarized in the following table:
| Antigen | CDC-1 | CDC-2 | CDC-3 | CDC-4 | CDC-5 | CDC-6 | CDC-7 | CDC-8 | CDC-9 | CDC-10 | CDC-11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Homoge neous/rim | Speckled/SS-B | Speckled | RNP | Sm | Nucleolar | SS-A | Centro-mere | Scl-70 | Jo-1 | PM-Scl | |
| nRNP/Sm | pos. | neg. | pos. | pos. | pos. | neg. | neg. | neg. | neg. | neg. | neg. |
| Sm | pos. | neg. | pos. | neg. | pos. | neg. | neg. | neg. | neg. | neg. | neg. |
| SS-A | neg. | pos. | pos. | neg. | neg. | neg. | pos. | neg. | neg. | neg. | neg. |
| Ro-52 | neg. | pos. | pos. | neg. | neg. | neg. | pos. | neg. | neg. | pos. | neg. |
| SS-B | neg. | pos. | pos. | neg. | neg. | neg. | neg. | neg. | neg. | neg. | neg. |
| Scl-70 | neg. | neg. | neg. | neg. | neg. | neg. | neg. | neg. | pos. | neg. | neg. |
| PM-Scl | neg. | neg. | neg. | neg. | neg. | neg. | neg. | neg. | neg. | neg. | pos. |
| Jo-1 | neg. | neg. | neg. | neg. | neg. | neg. | neg. | neg. | neg. | pos. | neg. |
| CENP B | neg. | neg. | neg. | neg. | neg. | neg. | neg. | pos. | neg. | neg. | neg. |
| PCNA | neg. | neg. | neg. | neg. | neg. | neg. | neg. | neg. | neg. | neg. | neg. |
| dsDNA | pos. | neg. | neg. | neg. | neg. | neg. | neg. | neg. | neg. | neg. | neg. |
| Nucleosomes | pos. | neg. | neg. | neg. | neg. | neg. | pos. | neg. | neg. | neg. | neg. |
| Histones | pos. | neg. | neg. | neg. | neg. | neg. | pos. | neg. | neg. | neg. | neg. |
| Rib. P-protein | neg. | neg. | neg. | neg. | neg. | neg. | neg. | neg. | neg. | neg. | neg. |
| M2 | neg. | neg. | neg. | neg. | neg. | neg. | neg. | pos. | neg. | neg. | neg. |
The specificity of these sera was determined at the "Center for Disease Control" by immunofluorescence patterns (substrate: HEp-2 cells and primate liver), the results of double immunodiffusion or counter immunoelectrophoresis (the sera are not in any case monospecific).
Measurement range: The EUROLINE is a qualitative method. No measurement range is provided. The titre limit is given at a dilution of 1:101.
Cross reactions: The high analytical specificity of the test system is guaranteed by the quality of the antigen substrates used (antigens and antigen sources). This EUROLINE specifically detects IgG class antibodies to Mi-2, Ku, nRNP/Sm, Sm, SS-A, Ro-52, SS-B, Scl-70, PM-Scl100, Jo-1, CENP B, PCNA, dsDNA, nucleosomes, histones, ribosomal P-protein and AMA M2. No cross reactions with other autoantibodies have been found.
Inter- and intra-assay variation: The inter-assay variation was determined by multiple analyses of characterised samples over several days. The intra-assay variation was determined by multiple analyses of characterised samples on one day. In every case, the intensity of the bands was within the specified range. This EUROLINE displays excellent inter- and intra-assay reproducibility.
Sensitivity and specificity:
Mi-2: For the detection of autoantibodies against Mi-2 a sensitivity of 100% in comparison to the reference methods indirect immunofluorescence (substrate combination HEp-2 cells and frozen sections of primate liver) and/or the confirmatory test Myositis EUROLINE-WB [24] was determined using 35 serological pre-characterized samples of patients with myositis (anti-Mi-2 positive: n=6). The specificity was 100% for healthy blood donors (n=50).
Ku: For the detection of autoantibodies against Ku a sensitivity of 100% in comparison to the reference methods indirect immunofluorescence (substrate combination HEp-2 cells and frozen sections of primate liver) and/or the confirmatory test Myositis EUROLINE-WB was determined using 35 serological precharacterized samples of patients with myositis (anti-Ku positive: n=7). The specificity was 100% for healthy blood donors (n=50).
nRNP/Sm: For the detection of autoantibodies against RNP/Sm a sensitivity of 100% with reference to the ELISA method was determined using 22 samples of patients with MCTD (mixed connective tissue disease). The specificity was 100% for healthy blood donors (n = 50) and 96% in a panel of non-SLE rheumatic diseases (Sjögren`s syndrome n = 14, systemic sclerosis n = 18, polymyositis n = 25).
Sm: For the detection of autoantibodies against Sm a sensitivity of 100% with reference to the ELISA method was determined using 45 samples of patients with SLE. The specificity was 100% for healthy blood donors (n = 50) and 100% in a panel of non-SLE rheumatic diseases (Sjögren`s syndrome n = 14, systemic sclerosis n = 18, polymyositis n = 25).
SS-A: For the detection of autoantibodies against SS-A a sensitivity of 100% with reference to the ELISA method was determined using 14 samples of patients with Sjögren`s syndrome. The specificity was 100% for healthy blood donors (n = 50) and 95% in a panel of non-SLE rheumatic diseases (systemic sclerosis n = 18, MCTD n = 22).
Ro-52: For the detection of autoantibodies against Ro-52 a sensitivity of 100% with reference to the westernblot method was determined using 103 samples of patients with SLE and Sjögren`s syndrome (SLE n = 23, Sjögren`s syndrome n = 77 and neonatal lupus erythematosus n = 3). The specificity was 100% for healthy blood donors (n = 65). Antibodies against Ro-52 are not disease specific and can be detected in samples from patients suffering from myositis, systemic sclerosis and other rheumatic diseases (11-16), i.e. in 7 of 20 samples of systemic sclerosis patients autoantibodies against Ro-52 were detected.
SS-B: For the detection of autoantibodies against SS-B a sensitivity of 100% with reference to the ELISA method was determined using 14 samples of patients with Sjögren`s syndrome. The specificity was 100% for healthy blood donors (n = 50) and 97% in a panel of non-SLE rheumatic diseases (systemic sclerosis n = 18, MCTD n = 22).
Scl-70: For the detection of autoantibodies against Scl-70 a sensitivity of 100% with reference to the ELISA method was determined using 18 samples of patients with systemic sclerosis. The specificity was 100% for healthy blood donors (n = 50) and for a panel of non-SLE rheumatic diseases (MCTD n = 22, Sjögren`s syndrome n = 14, myositis n = 25).
PM-Scl: In 14 of 20 sera of patients with polymyositis, having a nucleolar-positive pattern in the indirect immunofluorescence (HEp-2-cells/primate liver), autoantibodies against PM-Scl were detected. The specificity was 100% for healthy blood donors (n = 50) and 99% in a panel of non-SLE rheumatic diseases (MCTD n = 22, Sjögren`s syndrome n = 14, systemic sclerosis n = 18).
Jo-1: For the detection of autoantibodies against Jo-1 a sensitivity of 100% with reference to the ELISA method was determined using 5 samples of patients with myositis. The specificity was 100% for healthy blood donors (n = 50) and 99% in a panel of non-SLE rheumatic diseases (systemic sclerosis n = 18, MCTD n = 22, Sjögren`s syndrome n = 14).
CENP B: In 19 of 20 sera of patients with systemic sclerosis, having a centromer-positive pattern in the indirect immunofluorescence (HEp-2-cells/primate liver), autoantibodies against CENP B (sensitivity 95%) were detected. The specificity was 100% for healthy blood donors (n = 50) and 97% in a panel of non-SLE rheumatic diseases (MCTD n = 22, Sjögren`s syndrome n = 14, myositis n = 25).
PCNA: In 13 of 20 patient sera, having a cyclin I-positive pattern in the indirect immunofluorescence (HEp-2-cells/primate liver), autoantibodies against PCNA were detected. The specificity was 100% for healthy blood donors (n = 50) and 99% in cyclin I-negative sera of patients with SLE (n = 83).
dsDNA: For the detection of autoantibodies against dsDNA a sensitivity of 94% with reference to the ELISA method was determined using 36 samples of patients with SLE. The specificity was 100% for healthy blood donors (n = 50) and for a panel of non-SLE rheumatic diseases (Sjögren`s syndrome n = 14, systemic sclerosis n = 18).
Nucleosomes: For the detection of autoantibodies against nucleosomes a sensitivity of 97% with reference to the ELISA method was determined using 34 samples of patients with SLE. The specificity was 100% for healthy blood donors (n = 50) and in a panel of non-SLE rheumatic diseases (Sjögren`s syndrome n = 14, systemic sclerosis n = 18).
Histones: For the detection of autoantibodies against histones a sensitivity of 78% with reference to the ELISA method was determined using 41 samples of patients with SLE. The specificity was 100% for healthy blood donors (n = 50) and 97% in a panel of non-SLE rheumatic diseases (Sjögren`s syndrome n = 14, systemic sclerosis n = 18).
Ribosomal P-protein: For the detection of autoantibodies against ribosomal P-protein a sensitivity of 82% with reference to the ELISA method was determined using 49 samples of patients with SLE. The specificity was 100% for healthy blood donors (n = 50) and in a panel of non-SLE rheumatic diseases (Sjögren`s syndrome n = 14, systemic sclerosis n = 18).
AMA M2: For the detection of autoantibodies against AMA M2 a sensitivity of 100% with reference to the ELISA method was determined using 36 samples of patients with primary biliary liver cirrhosis. The specificity was 100% for healthy blood donors (n = 50) and 99% in a panel of other liver diseases (autoimmune hepatitis n = 28, toxic liver damage n = 38, viral hepatitis B/C n = 69).
Reference range: The reference range was determined using a cohort of healthy blood donors (n = 50). All blood donors were negative.
Antigens:
Autoantibodies against Mi-2 bind to a multi-component complex of the cell nucleus. Molecular biological examinations revealed the main antigen with a molecular mass of 218 kDa, which contains histondeacetylase and shows "nucleosome remodelling" activity.
Antibodies against Ku are directed against a DNA-binding, nuclear heterodimer (70 und 86 kDa) that is involved in repairing dsDNA breaks, preventing the recombination of telomeric ends and regulating their length.
nRNP and Sm belong to a group of small ribonucleoproteins (snRNP, small nuclear ribonucleoproteins) which consist of low molecular weight RNA with a high uridine content (U-RNA) complexed with various proteins (molecular weights 9 - 70 kDa). The RNA component is termed U1 to U6, depending on its behaviour in chromatography. Besides the particular RNA, the particles of U-nRNP contain six different core proteins (B, B', D, E, F, G), U1-nRNP additionally contains particle-specific proteins (70K, A, C). Antibodies to U1-nRNP are directed against one or more of the particle-specific proteins 70K, A or C. In contrast, antibodies to Sm can also react with one or more core proteins. The U-nRNP particles are involved in splicing of the pre-mRNA (pre-messenger RNA) - they split off the non-coding mRNA sequences (introns) and insert the coding mRNA sequences (exons) to recreate the messenger RNA.
The native SS-A antigen is a small ribonucleoprotein composed of one of five RNA molecules (Y1, Y2, Y3, Y4 or Y5 RNA; 80-112 bases) and a 60 kDa protein. The SS-A band in the EUROLINE consists of the native SS-A antigen. A 52 kDa protein (52 kDa) is also associated with the SS-A/Ro complex, but whether this protein is a component of the SS-A/Ro complex is controversially discussed in the literature. Isolated antibody reactions with Ro-52 should not be evaluated as anti-SS-A positive or specific for SLE or Sjögren's syndrome, since they can occur in many different autoimmune diseases.
We recommend interpreting the EUROLINE with reference to the ANA screening test (HEp-2 cells/primate liver) as follows:
| IIFT | EUROLINE | Result | |
|---|---|---|---|
| HEp-2 cells | Ro-52 (52 kDa) | SS-A (60 kDa) | |
| ANA negative | positive | negative | Anti-SS-A negative |
| ANA positive | positive | negative | Anti-SS-A negative |
| ANA positive | positive or negative | positive | Anti-SS-A positive |
It has been shown in various studies that anti-SS-A positive sera always contain antibodies against native SS-A (60 kDa protein) and may additionally exhibit antibodies against Ro-52. For example, in a Japanese study (EUROIMMUN) sera from 103 patients with SLE and Sjögren's syndrome (SLE n = 26, Sjögren's syndrome n = 77), which were characterized as anti-SS-A positive by double immunodiffusion, were investigated. 102 sera reacted with native SS-A, and 90 sera reacted additionally with the Ro-52 band. But no serum showed only a reaction with the Ro-52 band. This study demonstrates that antibodies against native SS-A can be reliably detected using the native SS-A. In rare cases and in suspected cases of neonatal lupus syndrome, the Ro-52 band may provide important supplementary information.
The SS-B antigen is a phosphoprotein with a molecular weigth of 48 kDa. It functions in the cell nucleus as a helper protein for RNA polymerase III.
The Scl-70 antigen has been identified as the enzyme DNA Topoisomerase-I. The molecular weight of the native antigen is 100 kDa. Originally, only a metabolic product of molecular weight 70 kDa was found in the western blot. The DNA Topoisomerase-I is situated in the nucleoplasm and, in a particularly high concentration, in the nucleolus. The enzyme participates in the replication and transcription of the DNA double helix.
The PM-Scl antigen is a complex of 11-16 polypeptides with molecular weights of between 20 and 110 kDa. The main antigens are two polypeptides of 75 and 100 kDa, which are known as PM-Scl75 and PM-Scl100. The two antigens are independent of one another and do not show any cross reactivity. PMScl is mainly localized in the nucleoli, but also occurs in the nucleoplasm. The function of the polypeptide complex has not yet been fully explained. It is suspected that PM-Scl plays a role in splicing of the 5.85 rRNA and some U-snRNAs.
The Jo-1 antigen is identical to Histidyl-tRNA synthetase, a cytoplasmic phosphoprotein with a molecular weight of 50 kDa. It joins the amino acid histidine in the cytoplasm to its corresponding tRNA.
Four different proteins were identified as centromere autoantigens: centromere protein-A (17 kDa), centromere protein-B (80 kDa), centromere protein-C (140 kDa) and centromere protein-D (50 kDa). All sera containing anti-centromere antibodies pre-characterized in indirect immunofluorescence tests are at least reactive with centromere protein B.
PCNA - proliferating cell nuclear antigen - with a molecular weight of 36 kDa is expressed cell cycle dependent. The active, trimeric form is a cofactor of DNA polymerases and takes part in the regulation of DNA repair. In indirect immunofluorescence on HEp-2 cells autoantibodies against PCNA produce a pattern called cyclin I. Half of the nuclei of all interphase cells display a bright, fine-granular basic fluorescence, whereby the nucleoli are excluded. The same fluorescence patterns can be seen in the other half, but the intensity is less by a factor of about 10.
Antibodies against DNA are distinguished into two different types: Antibodies against native, doublestranded DNA (dsDNA) and antibodies against denatured, single-stranded DNA. Antibodies defined as reactive with dsDNA recognize mainly epitopes in the deoxyribose phosphate backbone of the double helix. On the other hand, antibodies defined as reactive with ssDNA recognize polymers of purine and pyrimidine bases which are not accessible in the double-stranded form.
Nucleosomes are highly organized functional subunits of chromosomes consisting of histones (types H1, H2A, H2B, H3 and H4) and dsDNA. Their centre consists of a H3-H3-H4-H4 tetramer which is flanked on two sites by a H2A-H2B dimer each. The histone core particle is surrounded by two coils of the DNA double helix (146 base pairs in total). The nucleosomes are joined in a row in a string-of-pearls fashion, the DNA (linker DNA) is associated with the histone H1 in the region of the bond.
Histones are basic DNA-associated proteins with molecular weights from 11.2 kDa to 21.5 kDa. Their function is to stabilise the DNA double helix and also they might play a role in gene regulation mechanisms. Five distinct histone types exist: H1, H2A, H2B, H3, and H4. Histones are associated with DNA forming highly organized nucleosomal structures.
The ribosomal P-protein is composed of 3 proteins of the 60S-subunit of the ribosomes. These proteins are called P0 (38 kDa), P1 (19 kDa) and P2 (17 kDa). The main antigenic epitope is localized at the carboxy-terminus, which contains an identical sequence of 17 amino acids within all three proteins.
The M2 antigen system has been shown to comprise three biochemically related multi-enzyme complexes of the inner mitochondrial membrane which catalyze the oxidative decarboxylation of pyruvate, 2-oxoglutarate and branched-chain 2-oxoacids. Six proteins have been identified as M2 antigens: E2 (74 kDa) of the pyruvate dehydrogenase complex, Protein X (55 kDa) of the pyruvate dehydrogenase complex, E1 alpha subunit (45 kDa) of the pyruvate dehydrogenase complex and E1 beta subunit (36 kDa) of the pyruvate dehydrogenase complex; furthermore E2 (51 kDa) of the branched-chain 2-oxoacid dehydrogenase complex and E2 (51 kDa) of the 2-oxoglutarate dehydrogenase complex. The E2-enzymes are responsible for the transfer of Acyl groups to coenzyme A, protein X is a subunit of the pyruvate dehydrogenase complex with an unknown function.
Clinical Significance
Antibodies against nuclear antigens (ANA) are directed against various cell nuclear components (biochemical substances in the cell nucleus) [1, 2]. These encompass nucleic acids, cell nucleus proteins and ribonucleoproteins. The serological detection of autoantibodies against individual or several cell nuclear autoantigens using "multiparameter analysis" is an essential element in the diagnosis of autoimmune diseases, particularly rheumatic diseases [3, 4, 5]. The frequency (prevalence) of antinuclear antibodies in inflammatory rheumatic diseases is between 20% and 100% (in rheumatoid arthritis between 20% and 40%). Therefore, differential antibody diagnostics for the detection of antibodies against different nuclear antigens is indispensable in the identification of individual rheumatic diseases as well as useful in the diagnosis of further autoimmune diseases such as primary biliary cirrhosis (PBC) [3, 6, 7, 8, 9, 10].
The following inflammatory rheumatic diseases can be diagnosed using the ANA Profile et Mi-2 et Ku EUROLINE:
- systemic lupus erythematosus (SLE) [11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22]
- Sharp syndrome (mixed connective tissue disease = MCTD),
- Sjögren's syndrome (primary Sjögren's syndrome) [5],
- systemic sclerosis (systemic scleroderma, SSc) [1, 10],
- limited form of systemic sclerosis (CREST syndrome),
- myositis (poly-/dermatomyositis) [11, 14, 23, 24, 25, 26, 27, 28, 29],
- rheumatoid arthritis [5].
Overview
| Autoantibodies against | Autoimmune disease | Prevalence |
|---|---|---|
| Mi-2 | Adult or juvenile dermatomyositis Idiopathic myositis | 15%-30% or 10%-15% 8%-12% |
| Ku | SLE or systemic sclerosis (SSc) Myositis | 10% or 40@% |
| nRNP-Sm | Mixed connective tissue disease (MCTD) | 95% |
| Sm | SLE | 5%-40% |
| SS-A | Sjögren's syndrome or SLE Neonatal lupus erythematosus | 40%-95% or 20%-60�% -100% |
| Ro-52 | Sjögren's syndrome or SLE SSc or idiopathic inflammatory myopathy | 70%-90% or 40%-60 % or 20%-40% |
| SS-B | Sjögren's syndrome or SLE Neonatal lupus erythematosus | 40%-95% or 10%-20u% |
| Scl-70 | SSc Diffuse or limited form of SSc | 25%-75@%-65% or 5%-15% |
| PM-Scl | SSc including overlap syndrome Polymyositis/SSc overlap syndrome SSc (anti-PM-Scl75 positive) SSc (anti-PM-Scl100 positive) | 10%-20% or 5%-20% 7% |
| Jo-1 | Myositis (polymyositis/dermatomyositis) | 25%-35% |
| CENP B | SSc - limited form or SSc - diffuse form PBC | 80%-95% or 8%-30% |
| PCNA | SLE | 3% |
| dsDNA | SLE | 40%-90% |
| Nukleosomes | SLE | 40%-70% |
| Histones | Drug-induced SLE SLE or RA | 95%-100P% or 15%-50% |
| Ribosomal P-protein | SLE | 10% |
| AMA M2 | PBC or other chronic liver diseases SSc | Up to 96% or 30% 7%-25% |
Mi-2
Anti-Mi-2 antibodies (against nuclear helicase) have a diagnostic specificity of 95% for myositides, especially for dermatomyositis with hypertrophia of the nail folds. The prevalence of anti-Mi-2 antibodies is 15%-30% in adult dermatomyositis and 10%-15% in juvenile dermatomyositis. Some patients with anti-Mi-2 antibodies have polymyositis and, in rare cases, also inclusion body myositis [24]. Autoantibodies against Mi-2 can be produced very early and are often associated with a favourable disease course. Anti-Mi-2 antibodies have a high predictive importance in dermatomyositis (also with respect to adolescents). Antibodies against Mi-2 can also be detected in 8% to 12 % of patients with idiopathic myositis [24, 25, 26, 27, 28, 29, 30, 31, 32]. Dermatomyositides with anti-Mi-2 antibody presence are also associated with neoplasias (e.g. colon or breast carcinoma).
Ku
Autoantibodies against Ku (DNA-binding non-histone proteins) were first detected in polymyositis/systemic sclerosis overlap syndrome, but later also in other autoimmune diseases (SLE, SSc, MCTD and Sjögren's syndrome) with varied frequency (depending on the ethnic group). Antibodies against Ku occur with a prevalence of up to 10% in SLE, a systemic autoimmune disease belonging to the group of collagenoses, which predominantly manifests itself by the so-called butterfly rash [5, 13, 14, 22, 33]. 40% of patients with antibodies against Ku show symptoms of myositis or SSc, a chronic autoimmune disease with fibrosis of the skin (systemic sclerosis), the joints and inner organs such as oesophagus, lungs, heart and kidneys [1, 10, 25].
nRNP/Sm (U1-nRNP)
In nRNP/Sm (low-molecular-weight ribonuclear protein) the antibodies are directed exclusively against the core proteins A, C and 70 kDa of U1-nRNP [6, 7, 9, 16, 17]. High autoantibody titers against nRNP/Sm (with a sensitivity of 95% to 100%) are characteristic markers for Sharp syndrome, a multisymptomatic and multiform mixed connective tissue disease (MCTD) combining characteristics of rheumatoid arthritis, SLE, SSc and polymyositis. It has not yet been clarified if it is an independent disease. The autoantibody titer correlated with the activity of Sharp syndrome. Anti-nRNP/Sm antibodies can also be found in patients with SLE (15%-40%), SSC (2%-12%) and polymyositis (12%-16%) [13, 17, 35].
Sm
Sm (Smith antigen - name of the index patient) is a group of small ribonuclear proteins, which are involved in splicing pre-mRNA. They consist of RNA with high uridine content, U-RNA, and various proteins; molecular weight 9-70kDa. Autoantibodies against Sm are highly specific (over 90%) for SLE and can be found in 5%-40% of patients [11, 12, 13]. Together with antibodies against dsDNA, they can be considered pathognomonic for this condition. The prevalence in Caucasians amounts to 5%-10%. It is much higher in other ethnic groups, e.g. in Arabs (20%-42%), Chinese (around 30%) or Black Africans (around 30%) [15, 22, 33, 35, 36, 37]. For this reason, the prevalence given in many American studies is 20%-40%.
SS-A (native)
Autoantibodies against SS-A (soluble substance A or Sjögren's syndrome A or Robert antigen - name of the index patient; cytoplasmic protein complex consisting of one Y1-, Y2-, Y3-, Y4- or Y5-RNA molecule and a 60-kDa protein subunit) are directed against the 60 kDa protein subunit and associated with different autoimmune diseases [6, 7, 8, 9]. They most commonly occur in patients with Sjögren's syndrome (40%-95% of cases), but also in SLE (20%-60%) and primary biliary cirrhosis (20%), and occasionally also in autoimmune hepatitis and viral hepatitis [2, 12]. Apart from this, antibodies against SS-A can be found in practically 100% of cases of neonatal lupus erythematosus (neonatal LE syndrome). They are transmitted diaplacentally to the foetus and cause inflammatory reactions as well as a congenital AV block when the mother is anti-SS-A positive, in particular when anti-SS-B (Anti-La) are also present [11, 38, 39, 40]. Differentiation of anti-SS-A antibodies from those against the so-called Ro52 antigen (52 kDa protein, RING dependent E3 ligase) is of decisive diagnostic importance, since antibodies against Ro52 are not disease-specific, but are also detected in myositis, systemic sclerosis, other collagenoses, neonatal lupus erythematosus, primary biliary cirrhosis, autoimmune hepatitis and viral hepatitis [35, 40, 41, 42, 43, 44, 45].
Ro52
Autoantibodies against Ro52 (52 kDa protein of the cell nucleus, RING dependent E3 ligase) occur increased with anti-SS-B, anti-Jo-1 and anti-SS-A autoantibodies [46]. Ro52 is not an integral component of the Ro/SS-A ribonuclear protein complex, as previously thought. Autoantibodies against Ro52 were first detected with high prevalence and diagnostic relevance in patients with Sjögren's syndrome and SLE, and later also in myositis and SSc [41, 47, 48]. Isolated antibodies against Ro52 are often detected together with antibodies against Jo-1 or PM-Scl100. The prevalence in healthy people is 0.5% and in patients with myositis (idiopathic inflammatory myopathy) in Europe around 30% [46]. Anti- Ro-52 autoantibodies also appear to play an important role in neonatal lupus and congenital heart block [38, 40, 48]. In this case, certain epitopes are probably associated with the complications during the pregnancy [38].
SS-B
Autoantibodies against SS-B (soluble Substance B or Sjögren's syndrome B or Lane antigen - name of the index patient; transcription termination factor in the nucleus for RNA polymerase III) are found almost exclusively in women (29:1) in Sjögren's syndrome (40-95%), SLE (10%-20%) and neonatal lupus erythematosus (75%) [12, 39, 40]. In Sjögren's syndrome, combined SS-A and SS-B antibodies mainly occur [2].
Scl-70
Autoantibodies against Scl-70 (enzyme DNA topoisomerase I, in the nucleoplasm and, in a particularly high concentration, in the nucleolus, play a role in the replication and transcription of the DNA double helix) are observed in SSc. The disease can manifest itself in two forms, which cannot always be clearly differentiated: the diffuse cutaneous and the limited cutaneous form [1, 6, 10, 23, 49]. Antibodies against Scl-70 are detected in 25%-75% of patients with SSc, whereby the prevalence is 40%-65% in the diffuse form and 5%-15% in the limited form.
PM-Scl
PM-Scl is an antigen complex consisting of 11 to 16 polypeptides with molecular weights of between 20 and 110 kDa. They are located predominantly in the nucleoli and are involved in the formation of ribosomal RNA. The main antigens are PM-Scl100 and PM-Scl75. These two antigens are independent of one another and do not show any cross reactions. It is assumed that PM-Scl is involved in splicing of the 5.85 rRNA and some U-snRNA. The specificity is 99% (PM-Scl100) and 98% (PM-Scl75) and the sensitivity is 6.6% and 11.8%. Anti-PM-Scl antibodies are detected in 18% of patients with polymyositis/systemic sclerosis overlap syndrome. In these patients, the autoantibodies are generally directed against both main antigens, namely PM-Scl75 and PM-Scl100. If progressive systemic sclerosis is present, antibodies against PM-Scl75 show a prevalence of almost 10% and those against PM-Scl-100 a prevalence of 7% [5, 6, 10, 23, 50].
Jo-1
Antibodies against Jo-1 (cytoplasmic histidyl-tRNA synthetase) are found in polymyositis and dermatomyositis with a prevalence of 25% - 35% [9]. They are often associated with a concurrent interstitial fibrosis of the lung/fibrous alveolitis.
CENP B
Autoantibodies against centromeres (four different proteins: CENP A, B, C, D: centromere protein A with 17 kDa, centromere B 80 kDa, centromere C 140 kDa and centromere D 50 kDa) are associated with the limited form of SSc and occur in 80%-95% of patients [34, 51]. They are found in only 8% of patients with the diffuse form, but may also occur in 10%-30% of patients with primary biliary cirrhosis [49, 52, 53, 54].
PCNA
Autoantibodies against PCNA (proliferating cell nuclear antigen, cyclin I, helper protein for DNA polymerase delta with molecular weight of 36 kDa; key role in regulating the cell cycle: when it appears the S-phase begins. The protein is broken down by the middle of the G2 phase) are found in SLE with a prevalence of 3%. The specificity of antibodies against PCNA is very high (99%) [5, 9].
dsDNA
The detection of autoantibodies against deoxyribonucleic acid (DNA) is essential in the diagnosis of SLE [5, 6, 9]. Autoantibodies against DNA are divided into two different types: Autoantibodies against doublestranded, native DNA (dsDNA) and autoantibodies against single-stranded, denatured DNA. Antibodies against dsDNA react mainly with epitopes in the deoxyribose phosphate backbone of the double helix. On the other hand, autoantibodies defined as reactive with ssDNA recognise polymers of purine and pyrimidine bases which are not accessible in the double-stranded form. They may also react with epitopes of the deoxyribose phosphate backbone. The prevalence of autoantibodies against dsDNA amounts to 20%-90�pending on the detection method and disease activity [35]. Antibodies against dsDNA are also occasionally detected in patients with other autoimmune diseases and infections and, in rare cases, in clinically healthy people [55]. 85% of people in the latter group develop SLE within 5 years of initial detection of anti-dsDNA. However, SLE cannot be entirely excluded i f anti-dsDNA antibodies are not detected [56].
Nucleosomes
Autoantibodies against nucleosomes (functional subunits of chromosomes in the cell nucleus consisting of histones and dsDNA) can be detected in the serum of patients with SLE [56, 57]. Until recently, their relevance as a characteristic marker for SLE was limited since up to 70% of sera from systemic sclerosis patients reacted with conventionally prepared nucleosomes. Antibodies against nucleosomes detected using the new, highly purified nucleosome preparation from EUROIMMUN as the antigen have a specificity of almost 100% for SLE. However, with this test no reactions have been found with sera from blood donors or systemic sclerosis, Sjögren's syndrome or polymyositis patients [57, 58, 59, 60, 61].
Histones
Histones are nuclear proteins, namely type H1, type H2A, type H2B, type H3 and type H4, which form nucleosomes together with dsDNA. They are functional subunits of chromosomes in the cell nucleus. Autoantibodies can form against all five histone types. The most frequent are autoantibodies against H1 and H2B [5, 9, 62]. They are a constant find in drug-induced (procainamide, hydralazine, isoniazide and other) lupus erythematosus (95%). Around 50%-75% of patients treated with procainamide and 25%- 30% of those treated with hydralazine develop anti-nuclear antibodies without symptoms of SLE during long-term therapy. A third of these patients demonstrate antibodies against histones and after varied duration of therapy show clinical signs of drug-induced lupus erythematosus: polyarthralgia, pleuritis, pericarditis. The anti-nuclear antibodies persist for years after the drugs have been discontinued and the symptoms have abated [19, 20, 63]. Antibodies against histones also occur in around 50% of patients with non-drug-induced lupus erythematosus and in 5%-50% of patients with rheumatoid arthritis [54].
Ribosomal P-proteins
Ribosomal P-proteins (3 proteins of the 60S ribosomal subunit, referred to as P0 38 kDa, P1 19 kDa and P2 17 kDa; the main immunoreactive epitope is localised at the carboxy terminal, and in all 3 proteins consists of an identical sequence of 17 amino acids) are specific autoantigens in the diagnosis of SLE [7, 9, 12, 22]. In a multicentre study performed by EUROIMMUN serum samples from 360 SLE patients, 79 patients suffering from other collagenoses (SSc, Sjögren's syndrome, dermatomyositis/polymyositis, Sharp syndrome) and 206 healthy blood donors were investigated for ribosomal P-proteins (ARPA). ARPA were detected in 34 (9.4%) of the 360 SLE patients and 3 (12.5%) of the 24 patients with Sharp syndrome. In 2 of these 3 patients antibodies against dsDNA were detected, indicating an overlap with SLE. ARPA were not found in any of the patients with SSc, Sjögren's syndrome or dermatomyositis/polymyositis or in any of the healthy blood donors. In SLE the titer level of autoantibodies against ribosomal P-proteins did not correlate with the disease activity. The prevalence of ARPA was identical in SLE patients with or without CNS involvement, nephritis or hepatitis. ARPA may be detected more frequently in patients with other phenomena accompanying SLE, e.g. psychosis, although this is not statistically significant [64].
AMA M2
High titres of autoantibodies against M2 (AMA M2) are characteristic of primary biliary cirrhosis (PBC), whereby the E2 enzyme and protein X of the pyruvate dehydrogenase complex are the preferred antigens. PBC is an immune-mediated chronic inflammatory cholestatic liver disease of unknown aetiology. The disease is characterised by female predominance (>90 %) with most cases observed between the ages of 40 and 60. PBC incidence in different parts of the world is estimated to be 4 to 31 cases/million per year [9, 54, 65, 66].